> CO2 and Climate Change
Climate change - Greenhouse gases
Greenhouse gases are a natural part of the Earth's atmosphere, trapping radiation from the Earth's surface to keep the Earth's temperature warm enough to support life. The gases which trap radiation in the atmosphere – the so-called greenhouse gases – include water vapour, carbon dioxide, methane, nitrous oxide, perfluorocarbons, hydroflurocarbons, and sulphur hexaflouride.
The most common greenhouse gases are carbon dioxide and water vapour. Both are necessary to life on Earth, and exist in the atmosphere through natural cycles.
Carbon dioxide (CO2)
Carbon is stored on Earth in a number of major reservoirs: as carbon dioxide in the atmosphere; as carbon dioxide dissolved in water; as carbonate (limestone rocks and corals); as fossil fuels (coal, petroleum and natural gas, derived from once-living things); living plants; and dead organic matter (harvested wood and wood products, plant litter, humus in soil).
Carbon is continuously cycled between these reservoirs in the ocean, on the land, and in the atmosphere. This carbon cycle has been continuing naturally since plant life took hold on land about 400 million years ago.
Carbon dioxide is exhaled by humans and other animals and is used by plants in photosynthesis. Growing plants and the oceans act as carbon sinks, taking carbon dioxide from the atmosphere and storing the carbon. As plant material decomposes, the carbon is released back into the atmosphere, largely as carbon dioxide.
Given the right conditions soils, too, can build up stores of carbon, withholding carbon dioxide from the atmosphere. However, land-clearing and some agricultural practices result in a loss of carbon from soils and emissions of a range of greenhouse gases, including carbon dioxide.
The concentration of carbon dioxide in the atmosphere has been increasing because the natural carbon sinks of oceans, plants and soil have not been able to keep pace with the extra carbon dioxide emitted to the atmosphere through the burning of fossil fuels and other activities of the industrialised world.
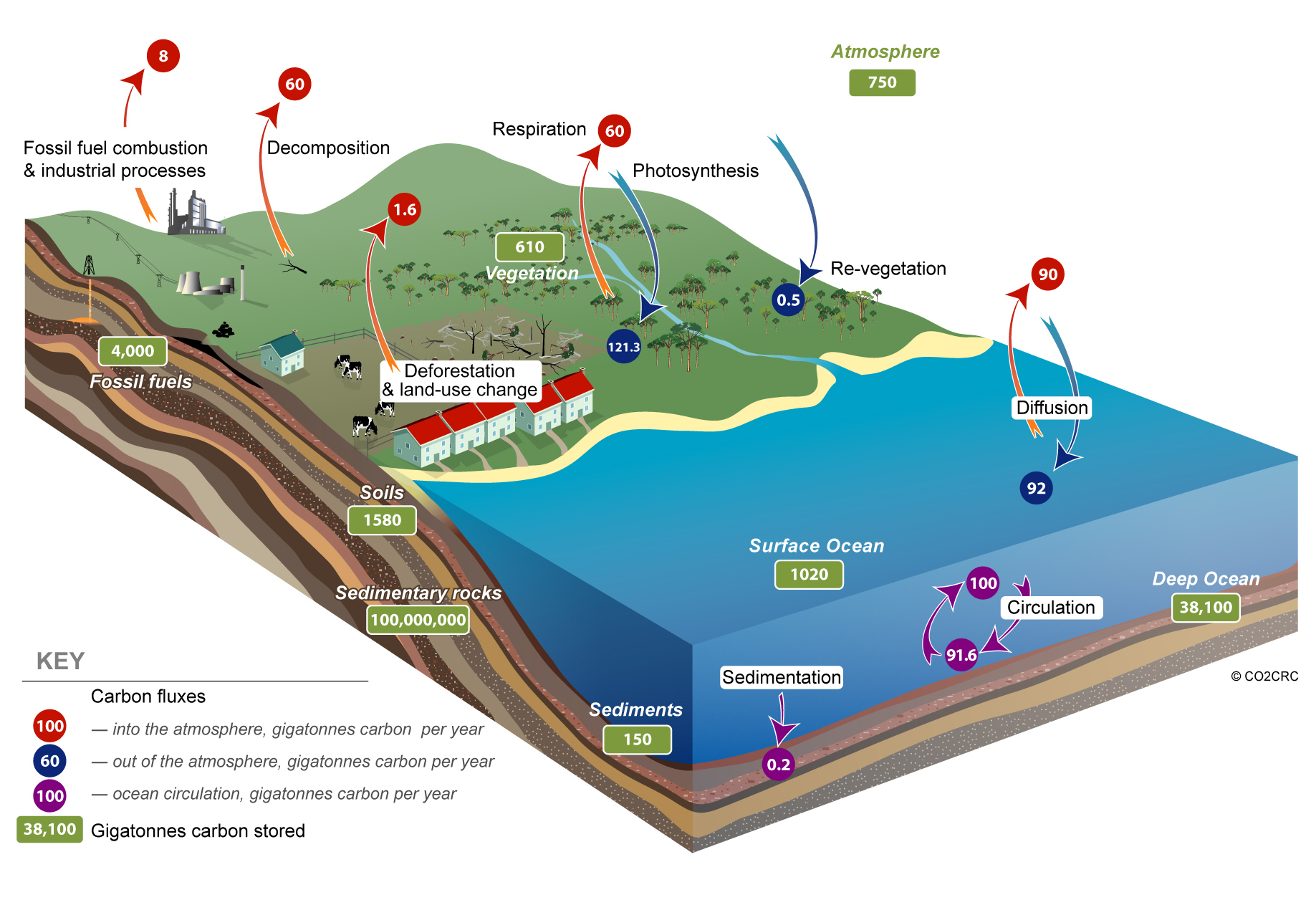
Not all of the carbon dioxide that has been emitted by human activities remains in the atmosphere. The oceans, responding to the higher concentrations of carbon dioxide in the atmosphere, have absorbed some of it. It is believed that of the about 7.1 billion tonnes of carbon released each year by human activities, about 3.2 billion tonnes remains in the atmosphere and 2 billion tonnes is absorbed by the world’s oceans. There is less certainty about the fate of the remaining 1.9 billion tonnes of carbon, but it is likely that it is absorbed by plants through processes such as the regrowth of some northern hemisphere forests and carbon dioxide fertilisation, through which plants – in the absence of water-, nutrient- or temperature-stresses – can grow more rapidly in an atmosphere high in carbon dioxide concentration.
The concentration of carbon dioxide in the atmosphere has increased from a pre-industrial level of about 280 parts per million to about 380 parts per million now.
The concentration of carbon dioxide in the Earth's atmosphere will continue to increase. Three quarters of the total increase is caused by burning fossil fuels, and even several centuries after emissions occur about one quarter of the carbon dioxide emitted remains in the atmosphere. Analysis by the Intergovernmental Panel on Climate Change indicates that even with the most rapid conceivable adoption of alternative technologies to cut use of fossil fuels, carbon dioxide levels in the atmosphere will continue to increase until about the end of this century, stabilising then at double pre-industrial levels. In other scenarios, assuming continuing world population growth and high reliance on fossil fuels, carbon dioxide levels in the atmosphere reach four times pre-industrial levels.
Water vapour (H2O)
Through processes of condensation, precipitation, inflitration, run-off, evaporation and transpiration, the Earth’s natural hydrologic cycle, water moves between the atmosphere (water vapour and clouds), the surface of the earth (liquid water, snow and ice, in oceans, rivers, lakes) and in the ground (groundwater, permafrost). Radiation from the sun drives the process, causing evaporation of water on the earth’s surface and transpiration from plants. As it is cooled, the water vapour in the atmosphere resulting from evaporation and transpiration condenses to become cloud or dew. When conditions are suitable, precipitation falls from clouds to the surface of the earth, and infiltrates the soil or flows to the ocean as runoff. Surface water (e.g., lakes, streams, oceans, etc.) evaporates, returning moisture to the atmosphere, while plants return water to the atmosphere by transpiration.
The average amount of water vapour – a significant greenhouse gas – in the atmosphere has increased since at least the 1980s. However, this increase is broadly consistent with the extra water vapour that the warmer air can hold. Rather than being the direct result of emissions of vapour through the activities of industrial society, changes to water vapour in the atmosphere represent the largest feedback from warming that affects climate sensitivity.
Methane (CH4)
Methane is formed when organic matter decomposes in the absence of oxygen. The digestive processes of livestock, waterlogged crops such as in rice cultivation, venting of natural gas, and waste decomposition in landfills are some of the major sources of methane emissions. Atmospheric concentration of methane has increased from a pre-industrial level of about 715 parts per billion to about 1774 parts per billion now. While this is much lower than the atmospheric concentration of carbon dioxide, each molecule of methane has 21 times the global warming impact of a molecule of carbon dioxide. As a result, the contribution of atmospheric methane to global warming is less than one-third that of carbon dioxide. The rate of growth of methane concentration in the atmosphere has decreased since the early 1990s.
Nitrous oxide (N2O)
The concentration of nitrous oxide in the atmosphere has risen from about 270 parts per billion in pre-industrial times to about 319 parts per billion now. Nitrous oxide breaks down very slowly in the atmosphere (over about 120 years) and is a potent greenhouse gas, with each molecule having about 310 times the impact of a molecule of carbon dioxide. However, because the concentration of nitrous oxide in the atmosphere remains much lower than the concentration of carbon dioxide, the climate impact of nitrous oxide emissions to date is relatively modest.
Nitrous oxide is released naturally from a wide range of biological sources in soils and the oceans, but about one-third of all nitrous oxide emissions are the direct result of human activity, primarily in agriculture, particularly from use of fertilisers.
Perfluorocarbons, hydroflurocarbons, and sulphur hexaflouride
These are industrially produced chemicals with high global warming potentials but which exist at relatively low concentrations in the atmosphere. Emissions of these gases are covered by the Kyoto Protocol. Hydrofluocarbon emissions are primarily substitutes for ozone-depleting substances; perfluorocarbon emissions result from semi-conductor manufacturing and as a by-product of primary aluminium production; and most sulphur hexafluoride emissions stem from electrical transmission and distribution systems. Each of these gases has a high global warming potential, but they comprise only a small percentage of mankind’s greenhouse gas emissions.
Source: Cooperative Research Centre for Greenhouse Gas Technologies (CO2CRC) in Australian
<< Previous page
---
Next page >>
TOP
|




