> CO2 Storage:
There are three main ways in which the captured CO2 can be stored:
> In deep geological formations
> In deep ocean water
> In the form of Mineral Carbonates
Geological Formations
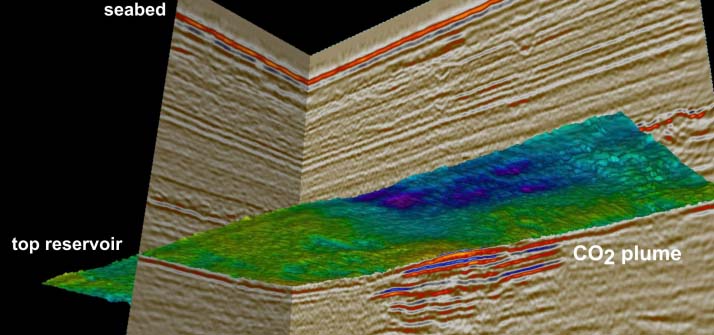
At present, injection into underground geological formations is the most promising and developed method. There are three main proposed underground storage sites: depleted oil and gas reservoirs, deep saline formations and deep unmineable coal seams. The CO2 is pumped into the reservoir in the supercritical phase. Supercritical CO2 behaves like a runny liquid. Various physical, e.g. impermeable caprock, and geochemical trapping mechanisms prevent CO2 from escaping to the surface. CO2 storage regulations will require that storage operations are rigorously monitored. Find out more about the monitoring of a storage site on the monitoring page.
Enhanced Oil Recovery
CO2 injected into oil reservoirs can also increase the oil production of the field. This process is called Enhanced Oil Recovery (EOR).
In the United States approximately 30 to 50 million tonnes of CO2 are injected annually into declining oil fields. This option is attractive for geological storage because the geological character of hydrocarbon reservoirs is well known, and because costs of injection may be partly offset by the sale of additional oil that is recovered.
The main disadvantages of using depleted oil fields are their geographic distribution which may not be ideal in relation to point sources, their limited capacity in comparison with the size of big point sources and the fact that the reservoir integrity may have been compromised during oil extraction. Also the subsequent burning of the additional oil so recovered through EOR will offset much or all of the reduction in CO2 emissions.
Unmineable Coal Seems
CO2 is adsorbed in the coal if the coal is permeable enough to allow CO2 to penetrate. In the process of absorption the coal releases previously absorbed methane, and the methane can be recovered. This methane can be used and the process of extracting useful methane from a coal seam when it is injected with CO2 is called ECBM (or enhanced coal bed methane).
The sale of the methane can be used to offset a portion of the cost of the CO2 storage. However as in EOR, burning the resultant methane would produce CO2 which would reduce some of the benefit of storing the original CO2.
Saline Formations
Formations filled with brine offer excellent potential for CO2 as they have the biggest capacity. However, relatively little is known about them, compared to oil fields and unlike storage in oil fields or coal beds no useful by-product will offset the cost of storage. More research is required on these formations but current research shows that several trapping mechanisms immobilise the CO2 underground, reducing the risk of leakage.
Ocean Storage
Storage in deep ocean water the least likely of the three storage options because large concentrations of CO2 kill ocean organisms as CO2 acts as an asphyxiant. Also, as CO2 reacts with the water to form carbonic acid, H2CO3, the acidity of ocean water would increase.
Mineral Carbonation
In mineral carbonation, captured CO2 is reacted with naturally occurring magnesium- (Mg) and calcium- (Ca) containing minerals. This process occurs naturally as the weathering of rock over geologic time periods.
The magnesium and calcium minerals are very abundant and are very stable. As a result, the re-release of CO2 into the atmosphere does not happen. However, these carbonation reactions are very slow under normal temperatures and pressures and to speed it up would need energy. The reaction rate can be made faster, by reacting at higher temperatures and pressures, or by pre-treatment of the minerals. If this process can be perfected it would offer a great storage solution for countries such as Oman because they have huge quantities of magnesium and calcium minerals in the form of ophiolite.
At NCCCS we are also working on processes to carbonate industrial waste materials and then utilising these carbonated waste materials in building materials.

|

|
Source: The Nottingham Centre for Carbon Capture and Storage
<< Previous page
---
Next page >>
TOP
|





