> Capture from air
CO2 Capture from the Air for Fuel or Storage
January 12, 2009
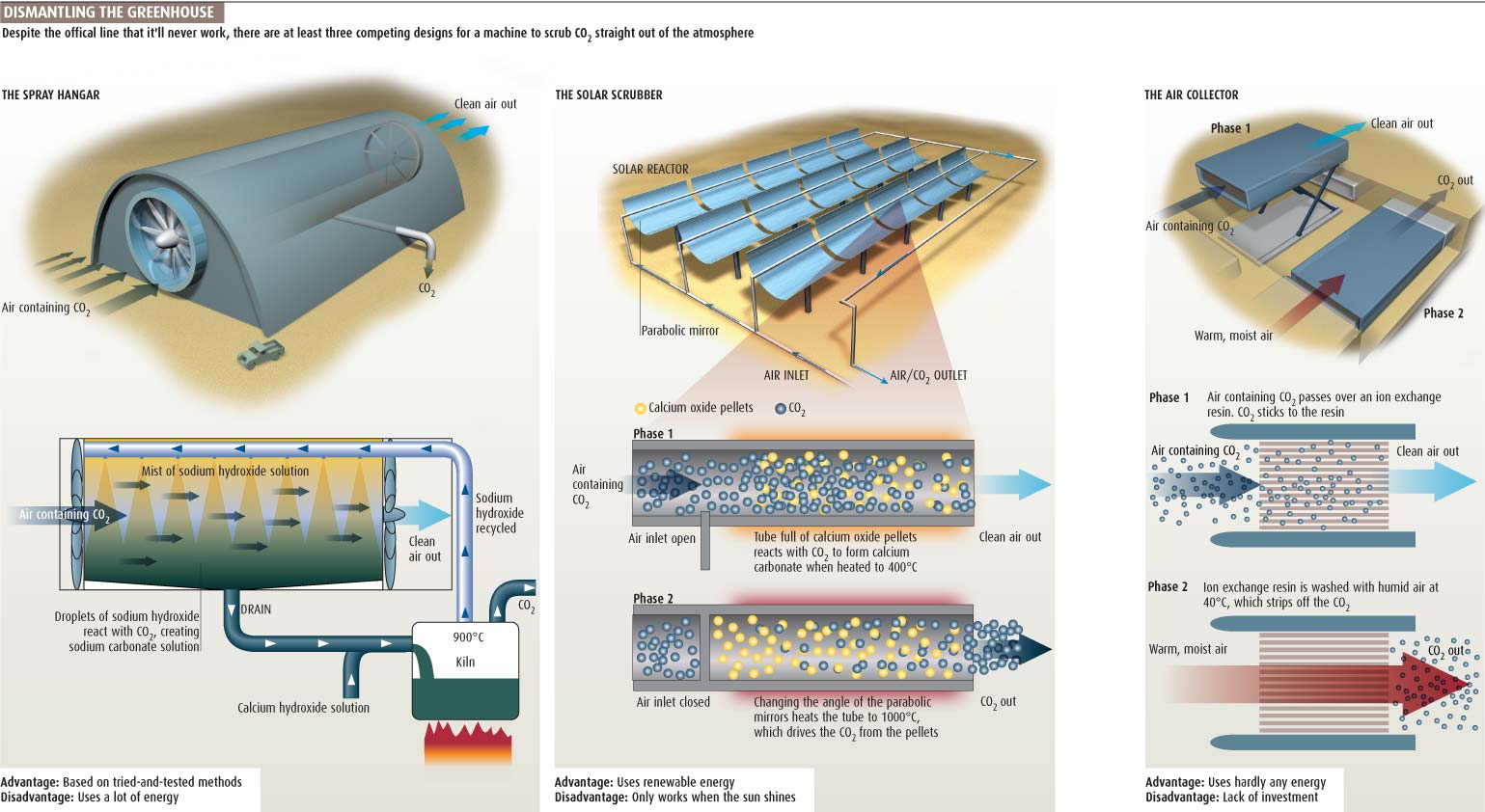
CO2 Capture from the Air Technology
Capturing CO2 gas is not difficult. In fact, in the near future we are likely to be extracting quantities of the stuff from the flue gases of power plants and factories, and stashing it deep underground. In 2005 a special committee of the Intergovernmental Panel on Climate Change concluded that this strategy - known as carbon capture and storage or CCS - is likely to have a key role in tackling climate change. An entire industry, well supported by governments and energy companies, has since sprung up to make CCS a reality.
Once CO2 gets out into the open air, however, capturing it gets trickier. Levels in the atmosphere are around 1/100th of its concentration in flue gases, and based on this the IPCC panel concluded that capturing CO2 directly out of the air was impractical in terms of the energy that would be required.
Klaus Lackner of Columbia University in New York City begs to differ. A particle physicist by training, he has been pushing air capture for years. He has worked out the theoretical minimum energy required to remove CO2 from air, and says that the IPCC panel - of which he was a member - got its numbers wrong. "It takes more energy to extract CO2 from air than from flue gas, but the difference is quite small," he says.
In mid-2008 he and his colleague Allen Wright were granted patents on a scrubber made of a plastic that spontaneously grabs CO2 from the air. Their device collects just a few tens of kilograms of CO2 a day from air blowing through the gaps between vertical sheets of the plastic. But with two years' work and, say, $20 million in venture capital - not easy to come by right now - Lackner says he could build a model that removes a tonne a day and would fit in a standard shipping container. Units like this might be of immediate interest to companies that have to buy in CO2, and Lackner suggests that millions of them could eventually be deployed to suck CO2 from the atmosphere and help save us from global warming.
Lackner isn't the only one working on air capture. Lab-scale units have also been built by teams at the University of Calgary in Alberta, Canada, [spray tower varient in aircraft hanger: plan to halve the energy cost to capture CO2 and sequestering it to the vicinity of $100 per tonne.] and at the Swiss Federal Institute of Technology (ETH) in Zurich. [Variant on concentrated solar power, replace central boiler for heat to power conversion with CO2 solar reactor]
Keith and his student Joshuah Stolaroff built their first air-capture prototype three years ago, using a version of the tried-and-tested "spray towers" that remove sulphur dioxide from the smokestacks of coal-fired power plants. Like sulphur dioxide, CO2 is an acid gas that can be soaked up by a solution of the alkali sodium hydroxide.
Keith and Stolaroff's prototype is a 4-metre-high cylinder of heavy-duty cardboard lined with PVC. The air to be treated is blown in at the top, where it is sprayed with a fine shower of sodium hydroxide solution. This reacts with CO2 in the air to form droplets of sodium carbonate.
A full-scale scrubber might resemble an aircraft hangar, with fans at one end blowing air through a mist of sodium hydroxide from nozzles in the ceiling. Drains in the floor would collect the sodium carbonate solution.
European concenctrated solar power variant:
ETH's Steinfeld's reactor is a transparent tube filled with pellets of calcium oxide. In the table-top version the tube is a few centimetres high and an arc lamp replaces the sun. As the light heats the tube and its contents to 400 °C, air mixed with a small amount of steam is pumped in at the bottom and up through the pellets. At this temperature, the calcium oxide reacts with CO2 to form calcium carbonate. "By the time the air leaves, there is no CO2," says Steinfeld. "We go from 385 parts per million to practically zero."
In less than 15 minutes, the pellets are mostly converted to calcium carbonate. At that point, Steinfeld closes the intake valve and intensifies the light, raising the temperature in the reactor to 800 °C. This drives off the CO2 as a stream of pure gas, which can be sent for sequestering, and converts the calcium carbonate back into calcium oxide. The researchers have run their reactor through five cycles of absorption and release with no decline in performance. Steinfeld believes his device could be scaled up to take significant amounts of CO2 out of the atmosphere, though as yet he doesn't know how much it would cost per tonne.
The Lackner system:
The heart of the Lackner system is an ion exchange resin, a polymer impregnated with sodium hydroxide. The sodium ions are firmly attached to the polymer but the hydroxide is loose and easily displaced by CO2, which binds to the sodium to form sodium bicarbonate. The chemistry is essentially the same as in the Calgary device, says Lackner, but because of the enormous surface area of the resin sheets, the reaction goes much faster.
The resin has a second major advantage: it changes state when it gets wet, reducing its affinity for CO2. "So we can drive the [absorbed] CO2 off just by adding moisture," says Lackner.
CO2 from Air for Fuel
There are several other CO2 from air research and commercial projects, some with direct conversion to fuel. There is overlap with the Lackner and European project described above.
1. Department of Energy's Sandia National Laboratories uses concentrated solar energy to chemically 'reenergize' CO2 into carbon monoxide in its 'Sunshine to Petrol' project. The CO is then used to synthesize a liquid combustible fuel like gasoline, diesel, and jet fuel. Researchers have already shown proof of concept for their technique. They are now completing a prototype device, called the Counter Rotating Ring Receiver Reactor Recuperator, which uses solar energy to break down CO2. While this isn't going to produce fuel commercially tomorrow – Sandia researchers say it could be 15 or 20 years before that happens – it is an exciting and important move forward.
2. Los Alamos National Laboratory's 'Green Freedom' technology would extract carbon dioxide from the atmosphere and turn it into fuels. Air would be blown over a liquid potassium carbonate liquid to absorb the CO2, and then the CO2 would be extracted from the liquid and electrochemically separated to turn it into fuel. The Green Freedom system could use existing cooling towers, like those at nuclear power plants, which would eliminate the need to build additional structures for processing large volumes of air.
3. CO2-to-fuel technology at Carbon Sciences technology is based on natural organic chemistry processes that occur in all living organisms. Here, carbon atoms extracted from CO2 and hydrogen atoms extracted from H2O are combined, creating hydrocarbon molecules using biocatalysts and small amounts of energy. Using advanced nano-engineered biocatalysts, the technology lends itself to very large industrial scale production. The company plans to demonstrate the technology within the next several months with a prototype that can convert a stream of CO2 into an immediately flammable liquid fuel.
4. In the ELCAT (Electrocatalytic gas-phase conversion of CO2 in confined Catalysts) project, researchers at several European universities have shown the feasibility of gas-phase CO2 conversion in a catalytic process that recycles carbon dioxide into liquid hydrocarbons and alcohols. The technology, which has the potential to cut global CO2 emissions by 5 percent, could be ready for application in a decade.
5. The University of Nottingham's Centre for Innovation in Carbon Capture and Storage in the UK has successfully completed transforming CO2 into natural gas. The CICCS group has replicated the process in plants, capturing CO2, water, and solar light and transforming it into carbohydrates to create methane.
Source: Next Big Future
<< Previous page
---
Next page >>
TOP
|





