> Air Capture
What is Air Capture?
“Air capture” is the extraction of carbon dioxide from atmospheric air in a closed-loop industrial process.
Capturing CO2 directly from the air allows emissions originating from any source to be managed with standardized scalable industrial facilities. Air capture is a tool for managing the buildup of CO2 in the atmosphere that drives climate change, and can serve as a compliment to other means that reduce emissions at their source. Air capture is unlike point-source carbon capture and storage (CCS) in that it enables industrial economies-of-scale to be applied to mobile and dispersed emissions sources.
Air capture requires an energy source, such as natural gas, concentrated solar power, or nuclear heat, and produces a stream of pure CO2 as its principal output. This CO2 can be used in industrial applications or stored in geological formations deep underground.

|
Carbon Engineering and other companies and research groups are developing air capture technologies using chemical-based absorbing solutions and specialized solid CO2-absorbing materials.
Carbon Neutral Fuels
A long-term application of air capture may be to produce carbon-neutral hydrocarbon fuels.
By using chemical processing methods to combine the CO2 produced from an air capture facility, with hydrogen split from water, hydrocarbon fuels (such as gasoline, jet-fuel, or diesel) could be manufactured from carbon originating in the atmosphere rather than in the earth’s crust. Once combusted, these fuels would simply return their CO2 to the atmosphere, leaving the overall concentration of CO2 un-altered.
Carbon-neutral hydrocarbon fuels are energy carriers; like electricity, they serve as a means to carry energy from its production source to point of use. Development of carbon-neutral hydrocarbon fuels would allow technologies which run best on liquid fuels, such as airplanes, to continue doing so without raising the concentration of CO2 in the atmosphere.
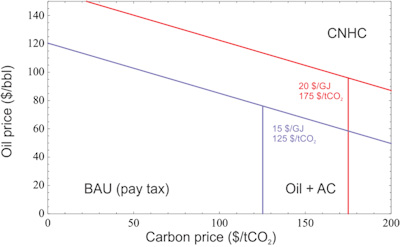
Carbon-neutral hydrocarbon fuels may be the most cost-effective means of producing liquid fuels under scenarios in which both the price of oil and the economic penalty for CO2 emissions, the “carbon price”, are relatively high.
|
Carbon Neutral Fuels: Given an oil price and a cost of carbon emissions, this plot shows regions in which fuel producers would prefer to sell fossil fuels and pay the carbon price (business as usual), produce fuels and negate their emissions with air capture, or use air capture to directly synthesize carbon-neutral fuels. CO2 and H2 prices are indicated for the two scenarios plotted.
The Carbon Engineering Design
CE’s patented technology integrates two processes: an air contactor, and a regeneration cycle, for continuous capture of atmospheric carbon dioxide and production of pure CO2.
These two processes work together to enable continuous capture of CO2 from atmospheric air, with energy (and small amounts of make-up chemicals) as an input, and pure CO2 as an output. The stream of pure CO2 can be sold and used in industrial applications and/or permanently sequestered (geologically stored) deep underground.
Air Capture Process
Our capture system brings atmospheric air containing CO2 into contact with a chemical solution that naturally absorbs CO2, in a device called a contactor. This solution, now containing the captured CO2, is sent to a regeneration cycle that simultaneously extracts the CO2 as a high-pressure pipeline-quality product while regenerating the original chemical solution, for re-use in the contactor.
Air Contactor
CE’s air contactor design captures carbon dioxide with a strongly alkaline hydroxide solution. This solution has been optimized to quickly absorb CO2 by careful selection of concentrations and additives.
We have developed and patented a unique contactor design that maximizes CO2 absorption by utilizing a large solution surface area, optimized air turbulence and mixing, and solution-refresh rates. Our contactor design enables us to capture industrial-scale quantities of CO2 using a cost-effective device with low solution pumping and fan energy inputs, and with minimal land use requirements.

|
Air Contactor Process
Air Contactor Components
Both the sodium hydroxide reactant used in our air contactor and the carbonate salt produced during the process are non-toxic, and are in fact used at low concentrations in the preparation of certain foods.
Regeneration Cycle
In CE’s regeneration cycle, the CO2-rich chemical solution from the air contactor is processed to release pure, compressed CO2, and also to re-generate the original capture solution for further use.
This cycle simultaneously extracts the CO2 as a high-pressure pipeline-quality product, while regenerating the alkaline hydroxide solution and returning it to the contactor.
Potassium/Calcium Capture and Recovery Cycle
Sodium/Iron Capture and Recovery Cycle
1. After CO2 is captured in the air contactor, it forms a chemical known as carbonate, which is carried to the regeneration cycle dissolved in solution. This solution is fed into a device called a crystallizer or causticizer which causes it to precipitate out of solution as solid carbonate.
2. Once the solid carbonate has been separated from the solution, it is sent to a kiln reactor. The kiln operates at about 900°C which causes the carbonate to decompose into an oxide such as CaO, NaO, or KO, during which pure CO2 is released as a gas. The kiln burns fuel, such as natural gas, in an oxygen environment to supply the heat needed to perform this reaction. This process also generates heat that is used to supply electricity for the rest of the air capture plant. The CO2 produced by burning the fuel mixes with the captured atmospheric CO2 and the entire stream is sent to a final “compression and clean-up” stage to produce pure, pipeline-quality CO2.
3. After the solids have released their CO2, they are then sent to a mixing tank where they react with water to re-form fresh hydroxide solution. This hydroxide solution is returned to the air contactor so that it can capture more CO2.
Regeneration Cycle Down-Selection
CE is evaluating the costs and performance of three related caustic-recovery processes based on cycles involving calcium, titanate, and iron.
Each cycle has been adapted from previous uses, which were focused on regenerating sodium hydroxide (CE’s capture chemical) while liberating CO2. All three cycles have R&D precedent and two have been used at full commercial scale; much work is being conducted by CE to evaluate these processes for application to air capture. CE has developed original process improvements, and is building pilot-scale equipment to optimize these recovery cycles to the unique task of atmospheric CO2 extraction. Mid-way through the current three-year R&D phase, we will select from among these processes based on cost estimation and process performance data. We will then integrate the selected regeneration cycle with our proprietary air contactor design, to form our full air capture facility.
Integration with Carbon-Free Energy
In CE’s lowest-technical risk ‘baseline’ design, all the input energy required onsite is supplied by natural gas. The carbon dioxide from gas combustion is also captured along with the CO2 extracted from the atmosphere, so that no new CO2 is emitted in the process.
In the longer term, we expect that carbon-free power will drive air capture. CE is building collaborations to conduct full-scale studies on integrating solar thermal or nuclear energy into its air capture system.
Source: Carbon Engineering - Calgary, Alberta, Canada
<< Previous page
---
Next page >>
TOP
|





