
|
What happens to the CO2
once in the storage reservoir?
|
Once injected in the reservoir, the CO2 will rise buoyantly filling the pore spaces below the cap rock. Over time, part of the CO2 will dissolve and eventually be transformed into minerals. These processes take place at different time scales and contribute to permanent trapping.
Trapping mechanisms
When injected in a reservoir, the CO2 fills the rock’s pore spaces, which in most cases are already filled with brine i.e. salty water.
As the CO2 is injected, the following mechanisms begin to come into play. The first is considered the most important and prevents the CO2 from rising to the surface. The other three tend to increase the efficiency and security of storage with time.
1. Accumulation below the cap rock (Structural trapping)
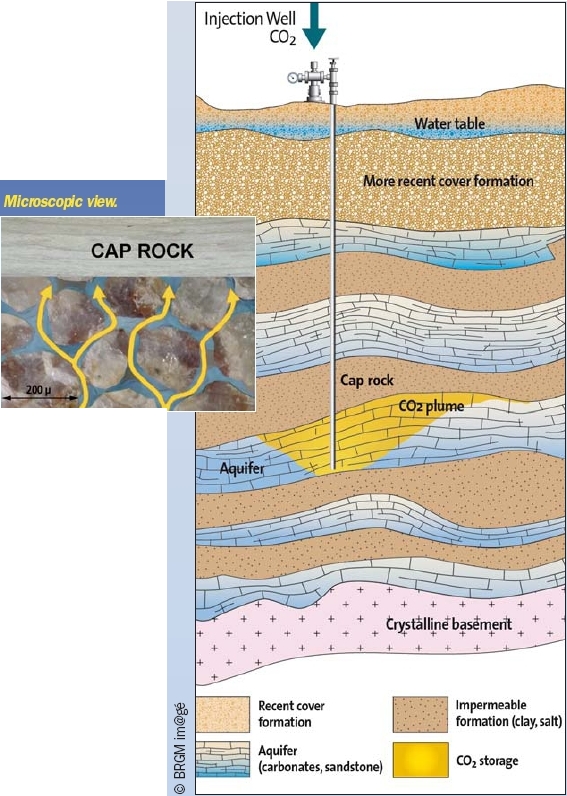
As dense CO2 is ‘lighter’ than water, it begins to rise upwards. This movement is stopped when the CO2 encounters a rock layer that is impermeable, the so-called ‘cap rock’. Commonly composed of clay or salt, this cap rock acts as a trap, preventing the CO2 from rising any farther, and leading to its accumulation directly beneath.
Figure 1 illustrates the upward movement of the CO2 through the pore spaces of the rock (in blue) until it reaches the cap rock.
|
Figure 1
The injected CO2, which is lighter than water, tends to rise and is stopped by overlying impermeable rocks.
|
2. Immobilization in small pores (Residual trapping)
Residual immobilization occurs when the pore spaces in the reservoir rock are so narrow that the CO2 can no longer move upwards, despite the difference in density with the surrounding water. This process occurs mainly during the migration of CO2 and can typically immobilize a few percent of the injected CO2, depending on the properties of the reservoir rock.
3. Dissolution (Dissolution trapping)
A small proportion of the injected CO2 is dissolved, or brought into solution, by the brine already present in the reservoir pore spaces. A consequence of dissolution is that the water with dissolved CO2 is heavier than the water without, and it tends to move downwards to the bottom of the reservoir. The dissolution rate depends on the contact between the CO2 and the brine. The amount of CO2 that can dissolve is limited by a maximum concentration. However, due to the movement of injected CO2 upwards and the water with dissolved CO2 downwards, there is a continuous renewal of the contact between brine and CO2, thus increasing the quantity that can be dissolved. These processes are relatively slow because they take place within narrow pore spaces. Rough estimates at the Sleipner project indicate that about 15% of the injected CO2 is dissolved after 10 years of injection.
4. Mineralization (Mineral trapping)
The CO2, especially in combination with the brine in the reservoir, can react with the minerals actually forming the rock. Certain minerals can dissolve, whereas others can precipitate, depending on the pH and the minerals constituting the reservoir rock (Fig. 2). Estimations at Sleipner indicate that only a relatively small fraction of the CO2 will be immobilized through mineralization after a very long period of time. After 10,000 years, only 5% of the injected CO2 should be mineralized while 95% would be dissolved, with no CO2 remaining as a separate dense phase.

|
Figure 2
Dense CO2 migrating upwards (light blue bubbles), dissolving and reacting with the grains of the rock, leading to precipitation of carbonate minerals on the grain boundaries (white).
|
The relative importance of these trapping mechanisms is site specific, i.e. it depends on the characteristics of each individual site. For instance, in dome-shaped reservoirs, CO2 should remain mostly in a dense phase even over very long timescales, while in flat reservoirs such as Sleipner, most of the injected CO2 will be dissolved or mineralized.
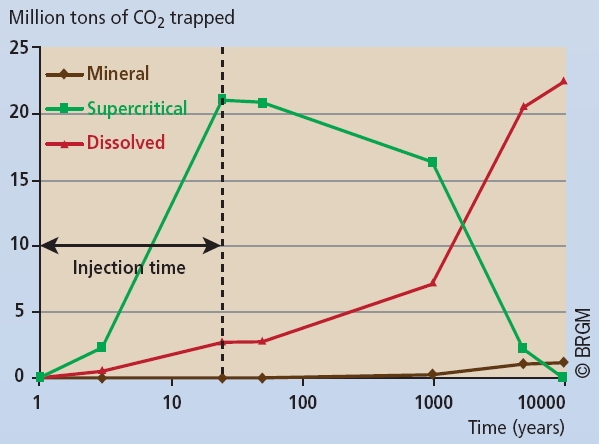
The evolution of the proportion of CO2 in the different trapping mechanisms for the Sleipner case is illustrated in Figure 3.
|
Figure 3
Evolution of the CO2 in its different forms in the Sleipner reservoir according to flow simulations. CO2 is trapped in supercritical form by mechanisms 1 and 2, in dissolved form by mechanism 3, and in mineral form by mechanism 4.
|
How do we know all this?
The knowledge of these processes comes from four main sources of information:
• Laboratory measurements: small-scale experiments for mineralization, flow and dissolution can be conducted on rock samples, giving insight into short-term and small-scale processes.
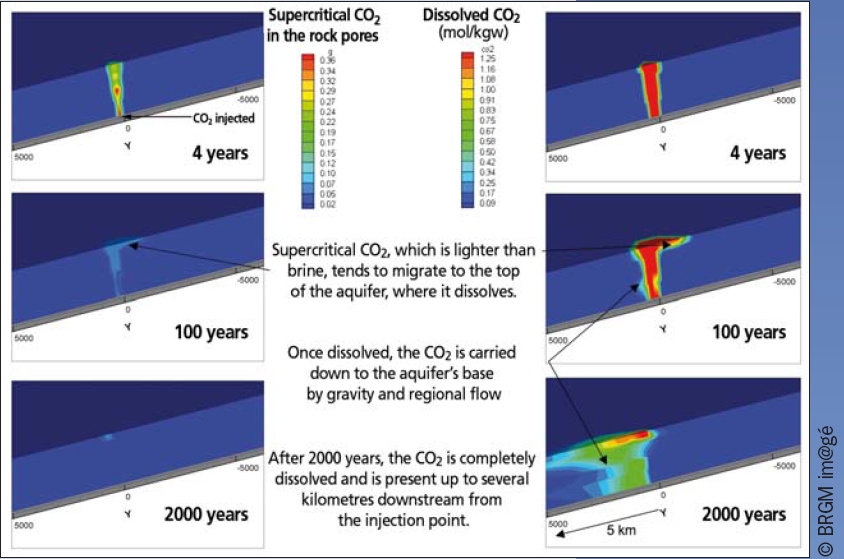
• Numerical simulations: computing codes have been developed that can be used to predict CO2 behaviour over much longer timescales (Fig. 4). Laboratory experiments are used to calibrate numerical simulations.
• The study of natural CO2 reservoirs, where the CO2 (generally of volcanic origin) has been trapped underground for long periods of time, often millions of years. Such a setting is referred to as a ‘natural analogue’*. These sites provide us with information on gas behaviour and the very long term consequences of the presence of CO2 in the underground.
• Monitoring of existing CO2 geological storage demonstration projects, such as Sleipner (offshore Norway), Weyburn (Canada), In Salah (Algeria) and K12-B (offshore The Netherlands). The results of the simulations in the short term can be compared with real field data and help refine the models.
|
Figure 4
3D modelling of CO2 migration in an aquifer, after the injection of 150,000 tons over 4 years in the Dogger aquifer in France.
Depicted here is the supercritical CO2 (left) and the dissolved CO2 in brine (right) 4, 100 and 2000 years after injection began. The simulation is based on field data and experiments.
|
Only by constantly cross-referencing and crosschecking these four sources of information is it possible to acquire reliable knowledge on all the processes occurring some 1000 m below our feet.
In conclusion, we know that the safety of a CO2 storage site tends to increase with time. The most critical point is to find a reservoir with a suitable cap rock above it that can withhold the CO2 (structural trapping). The processes related to dissolution, mineralization and residual trapping all work in favour of preventing CO2 from migrating to the surface.
Source: The European Network of Excellence on the Geological Storage of CO2
<< Previous page
---
Next page >>
TOP
|





