> CO2 Capture - separation technologies
Adsorption
Adsorption is based on a cyclical process in which carbon dioxide is adsorbed from a gas stream on to the surface of a solid, typically a mineral zeolite. The gas stream, with most of the carbon dioxide removed, is then emitted to the atmosphere. The solid is then purified in stages using differences in either pressure or temperature to remove the carbon dioxide and compress it for storage.
Adsorption occurs when a gas accumulates on the surface of a solid or a liquid, known as the adsorbent. The accumulated gas is called the adsorbate.

CO2 molecules are attracted to the surface of an adsorbent
In both pre-combustion and post-combustion capture, the flue gases are hot and wet. Developing an adsorbent-based system of capture requires a material that is cheap, environmentally friendly, water tolerant, impurity tolerant and works at high temperatures.
Adsorption systems operate in a three step cycle: adsorb CO2, purge (remove impure gases) and evacuate (remove/desorb CO2).
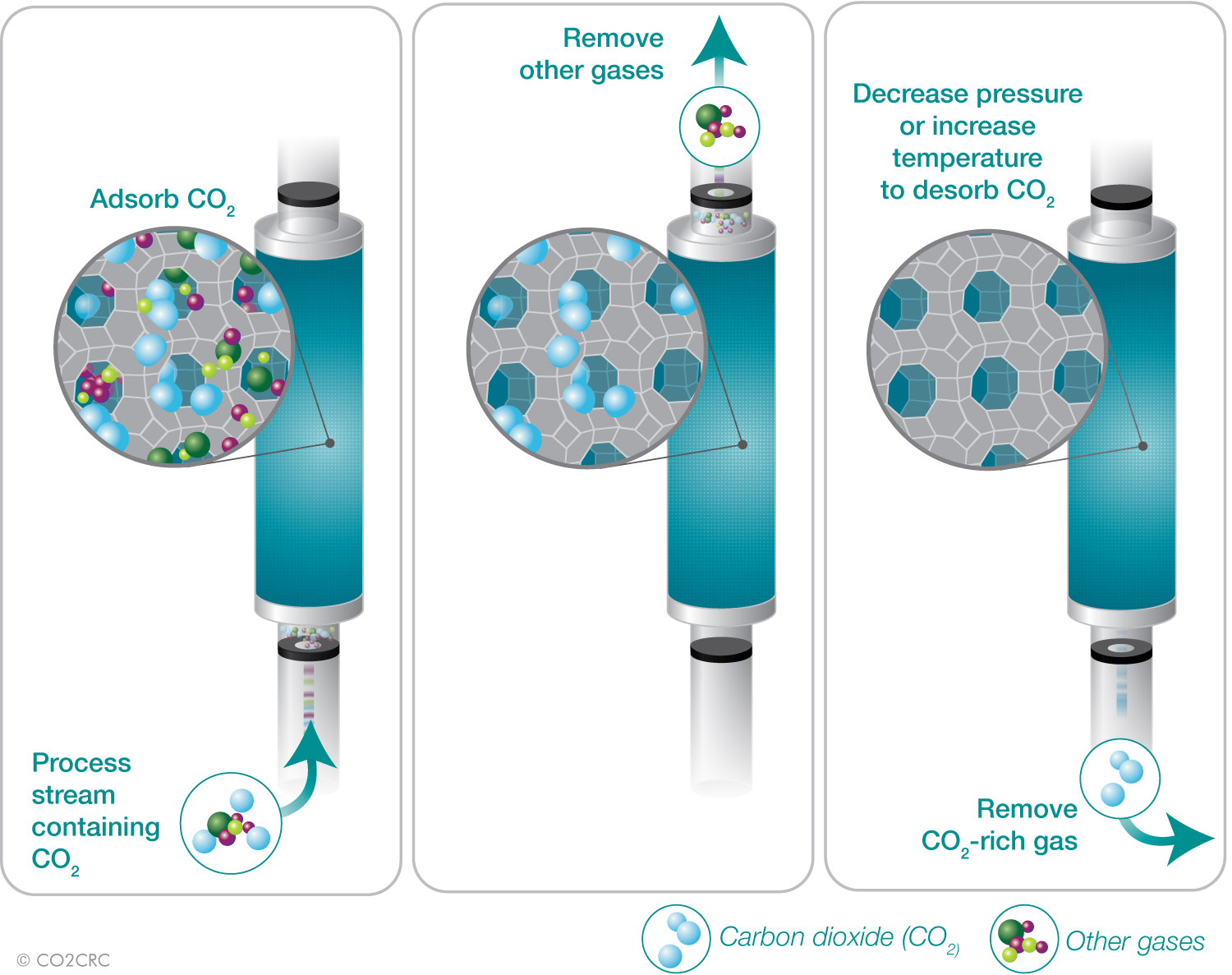
Principles of adorption. Click to enlarge.
>> View Flash animation of the adsorption and desorption cycles
In a typical vacuum swing adsorption process arrangement, optimal energy efficiency can be achieved using three adsorbent beds. If there are only one or two beds, there are idle steps where the pump is waiting for a bed to become available before being connected. This wastes time and energy.
In the three-bed adsorption process animation, this arrangement of three beds is shown going through the three basic steps and then a repressurisation stage. For simplification the process is shown separating CO2 from N2.
>> Feed – the flue gas goes into the adsorption bed, CO2 is adsorbed and N2 flows through.
>> Purge – CO2 is pumped back into the adsorption bed to flush out impure nitrogen trapped in spaces between the granules of the adsorbent.
>> Evacuate – the vacuum pump lowers the pressure and CO2 is removed from the chamber.
>> Repressurise – N2 flows back into the adsorption bed to re-pressurise the adsorption bed.
>> View Flash animation of three-bed adsorption process
Types of adsorption
CO2 is adsorbed through physical or chemical sorption.
Physisorption: The adsorbate is weakly bound onto the adsorbent by a combination of Van der Waals forces and electrostatic forces. No covalent bonds are formed and heat is released upon adsorption. Development of new adsorbents aims to increase the capacity of the adsorbent to attract CO2 by increasing the electric field and providing more surface area, without simultaneously increasing the amount of N2 adsorbed. It also aims to protect the adsorbent from enhanced water adsorption.
Chemisorption: There is covalent interaction of CO2 and the surface of the adsorbent which gives scope for much larger increases in adsorption capacity. Development of adsorbents aims to improve selectivity towards CO2 and ability to operate at high temperatures In inorganic-organic hybrids, functionality can be added to the hybrid to improve the sorption.
There are a number of process for desorption (extracting the CO2 from the adsorbent)
Adsorbent materials
Types of adsorbents include metal organic frameworks (MOFs), other organic-inorganic hybrids, zeolites and mesoporous carbons, in addition to conventional adsorbents such as silica gels and aluminas. New materials are being developed and tested and equipment is being designed to evaluate these new materials.
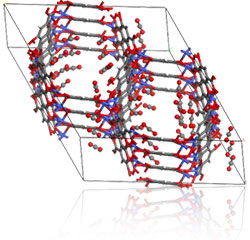
Metal Organic Frameworks (MOF)s
These are metal ions linked by organic bridging to form a porous structure that can act as a molecular sieve or as a storage device for some gases. They are easy to synthesise, highly porous, thermally stable and can be made in large quantities from low-cost ingredients. They can be designed for a specific pore size and functionality can be added to the pores.
|
|
Zeolites
These are hydrated aluminosilicate minerals which form regular, porous structures which act as a molecular sieve. They may be readily modified to include a large variety of metal cations through a simple ion-exchange process. These modifications lead to large changes in CO2 sorption capacity, selectivity and water tolerance.
|

|
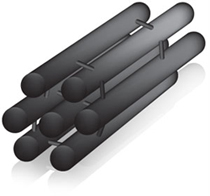
Mesoporous carbons
These are carbon structures (called carbon nanocages) containing nanopores (diameter between 2nm and 50 nm).
Functionality can be added to the pores.
|
|
Designing Functionality
Amine groups can be added to the pores of the adsorbent. These amine groups selectively attract CO2 molecules, which form a covalent bond with the amine group. This improves the adsorption capacity of the material
Desorption
Desorption (release of the CO2) is achieved by a number of methods:
> TSA – Thermal Swing Adsorption: the desorption is triggered by an increase in temperature. This is energy intensive and slow since the entire mass of adsorbent must be heated.
> VSA – Vacuum Swing Adsorption: the desorption of CO2 is triggered by creating a near-vacuum. One advantage is that this system will operate at near ambient temperature, so requires less energy. Another advantage is that the energy used is applied only to the CO2 and so it is thermodynamically more efficient than TSA.
> PSA – Pressure Swing Adsorption: the desorption is triggered by a decrease in pressure, usually from an elevated level to near atmospheric pressure.
> ESA – Electrical Swing Adsorption: the desorption is triggered by an applied voltage. This method has the advantage of being fast and requiring low energy.
> Different CO2 separation process configurations are possible depending on the temperature and pressure of the effluent gas stream.
Post-combustion Capture (ambient pressure flue gas)
CO2 separation from: N2, H2O (trace O2, NOx, SOx)
> Vacuum Swing Adsorption at Ambient Temperature (~30°C)
> Vacuum Swing Adsorption at Elevated Temperature (~110°C)
> Thermal Swing Adsorption at Ambient Pressure (1atm)
Precombustion Capture (high pressure synthesis gas)
CO2 separation from: H2, H2O, CO, (trace O2, S-gases)
> Pressure Swing Adsorption at Ambient Temperature (~30°C)
> Pressure Swing Adsorption at Elevated Temperature (~110°C)
Working capacity
In researching the effectiveness of various adsorbents, measurements are taken to determine the working capacity of the adsorbent. These measurements are taken under constant temperatures (the curve is an isotherm). The working capacity for a gas is the difference in adsorption capacity and desorption capacity.
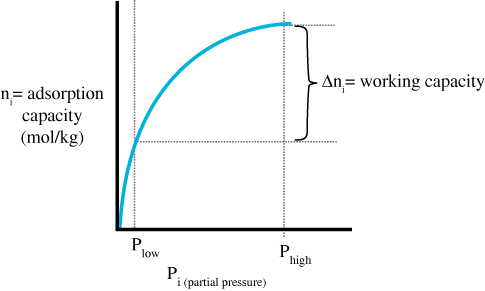
The adsorbent needs to have a good working selectivity (ratio of CO2 working capacity to N2 working capacity).
Objectives for the development of adsorbents and adsorbent processes can be summarised as follows:
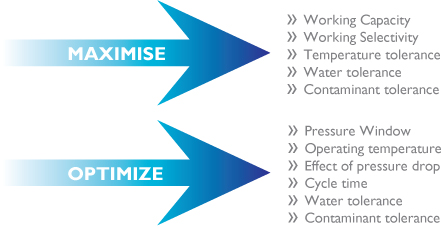
Calculating the amount of adsorbent and designing equipment
In order to design an adsorbent bed, the volume of adsorbent needs to be calculated. The bulk density of the adsorbent, which can range from 500-800 kg/m3, determines the volume occupied. Conventional adsorbents are pelletized and packed into vessels.

The adsorption/desorption equipment must be designed with a particular cycle time in mind, the cycle time being how long it takes for adsorption and desorption to occur. Typically, in order to capture 1 tpd CO2 with a cycle time of one minute, the plant would need 300 kg adsorbent. Therefore, a 10,000 tpd plant will need 3m kg adsorbent. At $5/kg, the total adsorbent cost is $15m. Improving the working capacity of the adsorbent by 10% will save $1.5m and reducing cycle time by 10% will save $1.5m. These are areas of research focus in developing new adsorbents and adsorbent systems.
Source: Cooperative Research Centre for Greenhouse Gas Technologies (CO2CRC) in Australian
<< Previous page
---
Next page >>
TOP
|





