> CO2 Capture - separation technologies
Membranes
Membranes, made of polymers or ceramics, can be used to effectively sieve out carbon dioxide from gas streams. The membrane material is specifically designed to preferentially separate the molecules in the mixture. A range of configurations exists either simply as gas separation devices or incorporating liquid absorption stages. This process has not yet been applied on a large scale and there are challenges related to the composition and temperature of the flue gases.
Membranes are used to separate CO2 from other gases (gas separation membranes) and to allow CO2 to be absorbed from a gas stream into a solvent (membrane gas absorption ). Other membranes being developed are facilitated transport membranes. There are a range of membranes types for these processes.
Membrane Gas Absorption
A membrane can be used with a solvent to capture the CO2. The CO2 diffuses between the pores in the membrane and is then absorbed by the solvent. The membrane maintains the surface area between gas and liquid phases. This type of membrane is used when the CO2 has a low partial pressure, such as in flue gases, because the driving force for gas separation is small.
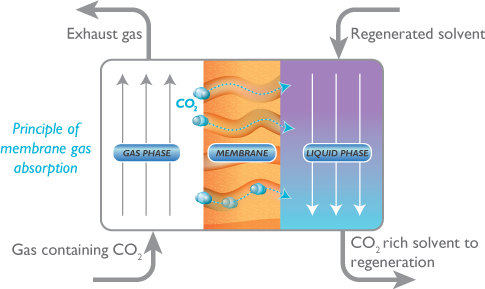
In the diagram above, the porous membrane allows gases to come into contact with the solvent. Only CO2 is absorbed because of the selectivity of the solvent. The membrane itself does not separate the CO2 from other gases, but rather maintains a barrier between the liquid and gas with permeability through the pores.
In a traditional solvent absorption process, the liquid and the gas are together, which leads to flow problems such as foaming and channeling. The physical separation of the gas flow from the liquid flow eliminates these problems.
Using a compact membrane can reduce the size of the equipment required to absorb the CO2. Reseach is focussed on developing appropriate solvents and optimising the reaction time.
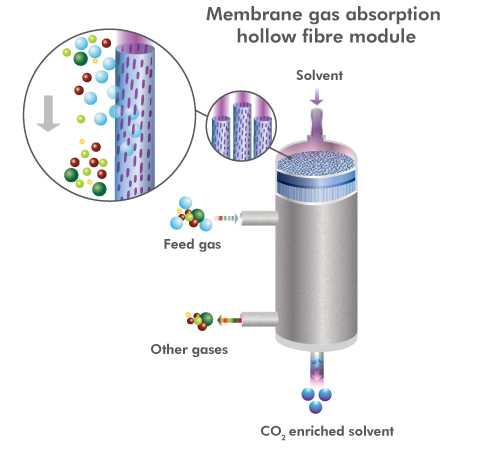
Hollow fibre modules are one of the possible arrangements for reducing the size of capture equipment.
>> View Flash animation of membrane gas absorption
Gas separation membranes
The advantage of using gas separation membranes is that the equipment is much smaller and there is no solvent involved. At the current stage of development, the main cost is the energy required to create a large enough pressure difference across the membrane to drive separation.
A membrane acts as a semi-permeable barrier. The CO2 passes through this barrier more easily than other gases. In general, the rate at which a particular gas will move through the membrane can be determined by the size of the molecule, the concentration of gas, the pressure difference across the membrane and the affinity of the gas for the membrane material.
There are a number of mechanisms for gas separation in membranes:
> Knudsen diffusion: gas components are separated based on the difference in the mean path of the gas molecules.
> Molecular sieving: gas components are separated based on size exclusion, the size being the kinetic diameter of the gas molecules.
> Solution-diffusion: the gases are separated by their solubility within the membrane and their diffusions through the dense membrane matrix. This is the usual separation mechanism for polymeric membranes (rubbers, polyimides, cellulose acetate).
> Surface diffusion: gas molecules with higher polarity are selectively adsorbed onto the surface of the membrane and pass through the membrane by moving from one adsorption site to another (see adsorption).
> Capillary condensation
The most common are molecular sieving and solution-diffusion.
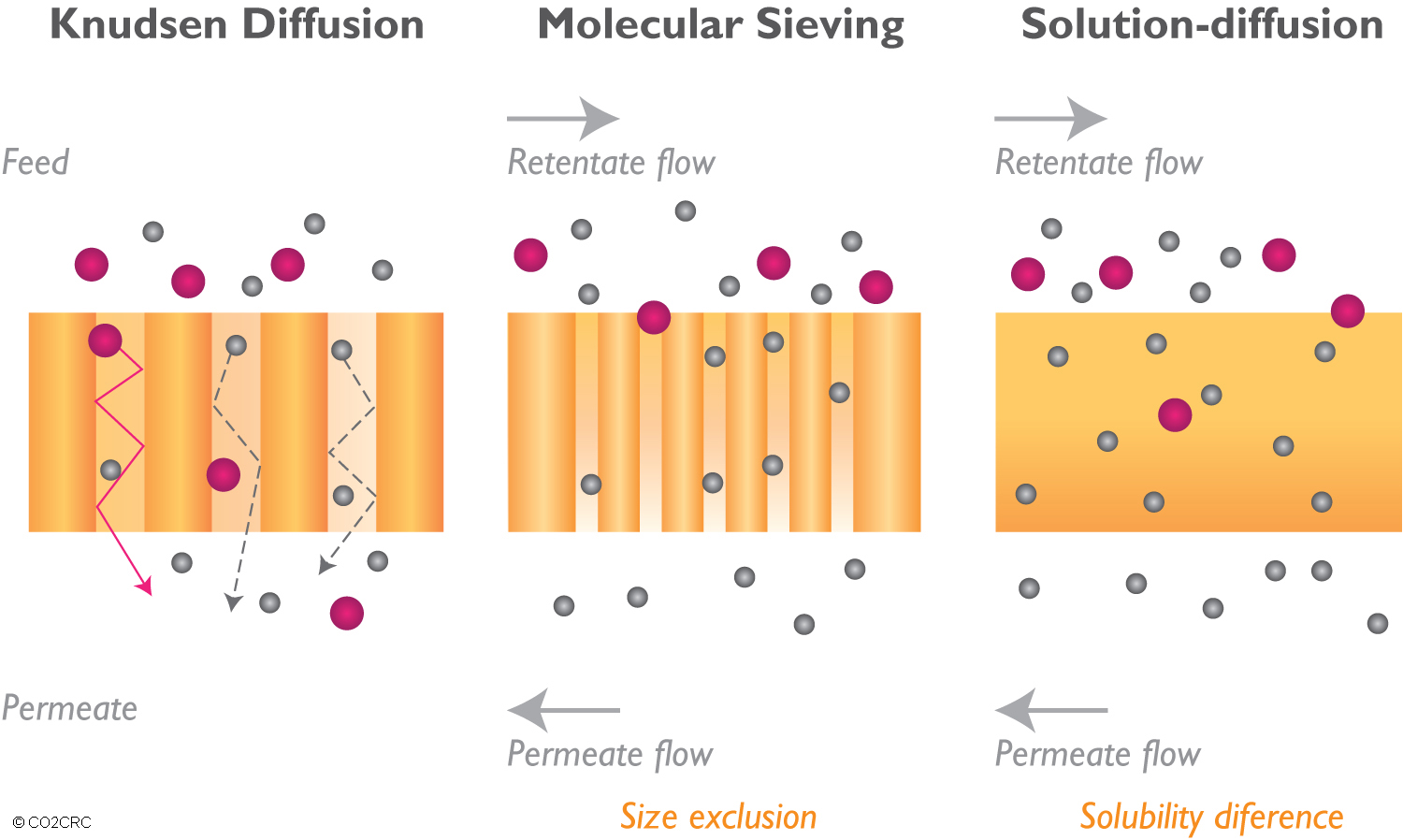
Schematic representation of three of the different possible mechanisms for membrane gas separation (after Scholes, Kentish and Stevens). (Click the image to enlarge)
Pore size and transport mechanism
The mechanism for transport across a porous membrane is in part dependent on the size of the membrane. For carbon porous membranes, this dependence on size is represented below.

Increasing pore size in nanometres.
Schematic view of membrane gas separation.
Facilitated transport membranes – new technology for CO2 capture?
Facilitated transport usually describes a biological process where molecules from the bloodstream are transported across cell walls into cells by specialized membrane proteins. Research into this process would involve developing molecules within membranes that aid CO2 passing through the membrane.
Membrane types
Development of new membranes has focused on increasing permeability and selectivity. In general, a highly permeable membrane has low selectivity and vice versa. This is described in the diagram below, with the upper bound known as Robeson’s upper bound.
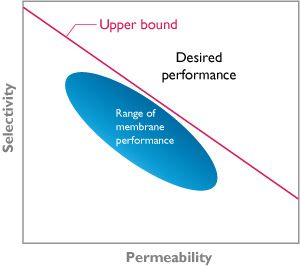
Robesons upper bound.
Polymeric membranes
Early membranes for CO2 separation were made from cellulose acetate. Composite membranes with a glassy (or hard) polymer segment and a rubbery polymer segment are amongst the new developments. Microporous polymeric structures and carbon membranes are also being developed.
The polymeric membranes do not operate well at high temperatures.
Inorganic membranes
The attraction of inorganic membranes is that they can operate at higher temperatures than the organic membranes.
Zeolites (aluminosilicates) act as molecular sieves.
Some membranes have functionalised pores which have a high chemical affinity for CO2. This increases permeability
Issues and limitations
> Polymeric membranes are proven in the capture of CO2 from natural gas
> Existing issues include:
- Plasticization
- Compaction
- Aging
- Competitive Sorption
- Fouling
> Pre-combustion capture is likely to be viable, particularly for hydrogen separation within membrane reactors
> Post-combustion capture offers the greatest challenge for membranes
Source: Cooperative Research Centre for Greenhouse Gas Technologies (CO2CRC) in Australian
<< Previous page
---
Next page >>
TOP
|





