> CO2 Capture - separation technologies
Absorption
Solvent absorption involves a cyclical process in which carbon dioxide is absorbed from a gas stream directed into a liquid, typically an amine. The gas stream, with most of the carbon dioxide removed, is then emitted to the atmosphere. The liquid is processed to remove the carbon dioxide, which is then compressed for storage. The resulting carbon-dioxide-free liquid is used again for absorption and the process continues. This technique is fairly widely used in a range of applications, but it needs a large amount of power to regenerate the solvent.
The absorption process is most commonly applied to post-combustion capture and in the main, the absorbents are liquid solvents.
In post-combustion systems, the flue gas needs to be cooled and impurities removed so that the solvent can efficiently absorb the CO2. The flue gases containing CO2 and N2 are then fed into a tower called the absorber tower. The flue gas comes into the bottom of the tower while the solvent is fed into the top of the tower. There is packing material in the tower, and the flue gas flows up through the packing, making contact with the solvent as it falls down. The CO2 is absorbed by the solvent as the flue gas rises so that the gas that comes out of the top of the tower contains very little CO2. Modifying the packing material to improve the absorption of CO2 is another area of research.
The solvent, with dissolved CO2, is then removed from the chamber. The N2 is released as it is not absorbed in the solvent. The recovery of CO2 from the solvent is called desorption. The usual process for recovery of CO2 from the solvent is temperature change. Other methods include pressure changes and the use of membranes with solvents.
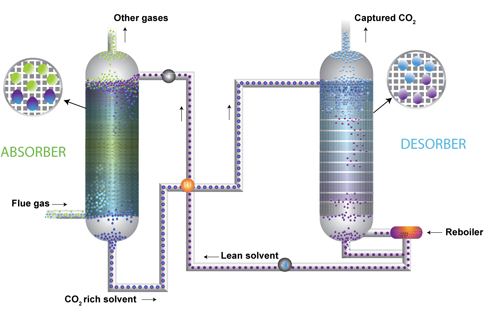
Solvent – based absorption capture
>> View Flash animation of the absorption and desorption cycle
Temperature changes
The loaded solvent passes into another tower where is it heated. As it falls down through the tower, the CO2 is desorpted (released) from the rich solvent at temperatures of 100o+ C. Water-saturated CO2 flows from the top of the tower into a tank which removes excess water. The solvent is recovered to absorb more CO2.
As all the solvent must be heated, not just the CO2, solvent capture of CO2 results in significant reductions in energy efficiency. Heat is necessary to regenerate the solvent and recover the CO2. Energy is also required to operate pumps and fans and to compress the CO2. This can amount to a significant energy penalty. Research aims to design systems which will re-use heat efficiently.
Types of solvents
A common solvent is monoethanolamine (MEA). Other solvents used are carbonates or bicarbonates. The volume of solvent is considerable. A 500MW generator would need approximately 6ML of solvent, about half in the absorption tower and the other half in the desorption tower.
Composition of flue gas
Black coal flue gas contains 71% N2, 12.6% CO2 and 11.1% water.
Brown coal flue gas contains 60% N2, 12% CO2 and 24% water.
Solvent recovery
Impurities such as nitrogen oxides (NOx) and sulphur oxides (SOx) can form heat stable salts and reduce the capacity of the solvent to absorb CO2. In addition, fly ash and soot also need to be removed. These impurities can be removed prior to absorption and often in coal-fired power plants there is already equipment in place as part of environmental controls which will remove most of the impurities. More of the oxides can be removed in a scrubber which can also double as the cooling equipment. As recovery of the solvent for re-use is an important element in the cost of capture, researchers working with new solvents target the effects of SOx and NOx on the new solvents and processes that may remove SOx and NOx in the one step.
CO2 solvent removal plant
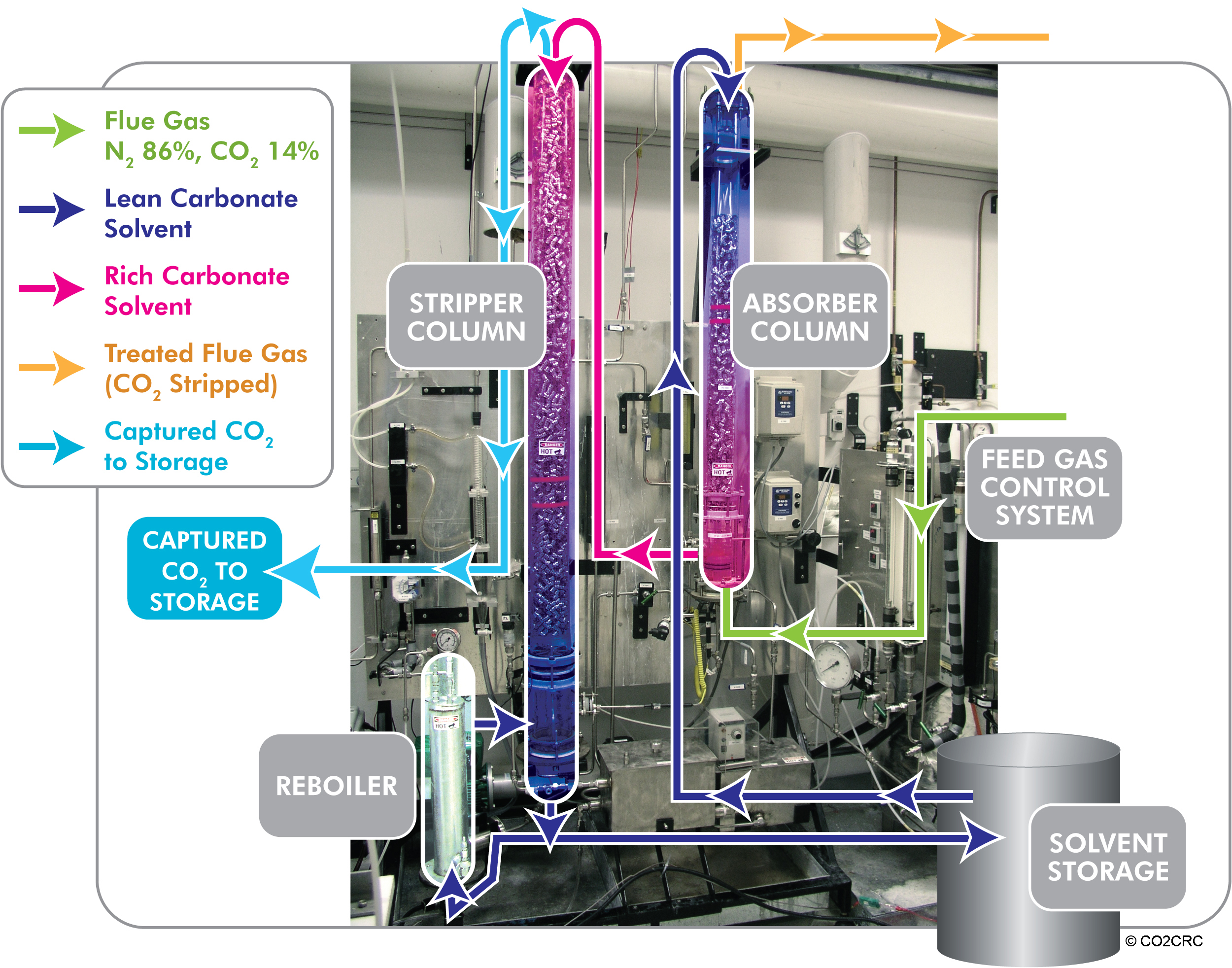
Source: Cooperative Research Centre for Greenhouse Gas Technologies (CO2CRC) in Australian
<< Previous page
---
Next page >>
TOP
|





