> CO2 Capture:
The first step in carbon capture and storage (CCS) is separating the carbon dioxide from other gases in the exhaust stream and, in the process, capturing the carbon dioxide (CO2).
CO2 capture can be applied to a variety of stationary sources of CO2, using a variety of CO2 capture technologies. The CO2CRC Capture Program researches, develops and demonstrates technologies that can reduce capture costs by 75 to 80 per cent.
CO2 capture applications
In a few instances, industrial processes emit fairly pure carbon dioxide which can be captured and separated relatively cheaply. Such processes include the manufacture of some fertilisers, natural-gas processing (where any carbon dioxide has to be separated from the natural gas to make it marketable) and cement manufacturing (the exhaust stream from modern cement plants have carbon dioxide concentrations of up to 50 per cent).
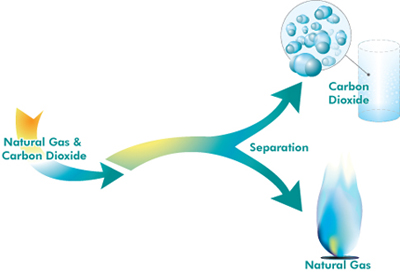
Schematic representation of CO2 separation from natural gas.
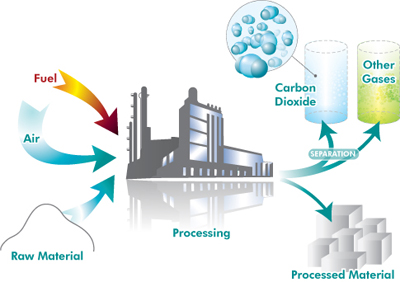
Schematic representation of CO2 capture from industrial processes.
Most of modern industrial society’s emissions of carbon dioxide come from coal-fired power stations, which can have flue-gas carbon-dioxide concentrations as low as 10 to 15 per cent. Separating such low concentrations of carbon dioxide from an exhaust stream is a complex and, with today’s technology, expensive process. CO2CRC is conducting extensive research into CO2 capture technologies to reduce this cost.
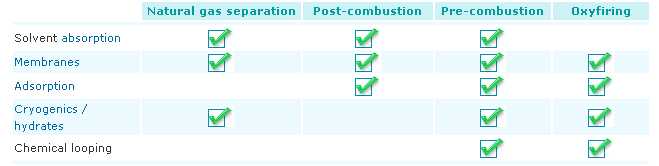
Five main technologies exist for separating carbon dioxide from a gas stream:
Solvent absorption involves a cyclical process in which carbon dioxide is absorbed from a gas stream directed into a liquid, typically an amine. The gas stream, with most of the carbon dioxide removed, is then emitted to the atmosphere. The liquid is processed to remove the carbon dioxide, which is then compressed for storage. The resulting carbon-dioxide-free liquid is used again for absorption and the process continues. This technique is fairly widely used in a range of applications, but it needs a large amount of power to regenerate the solvent.
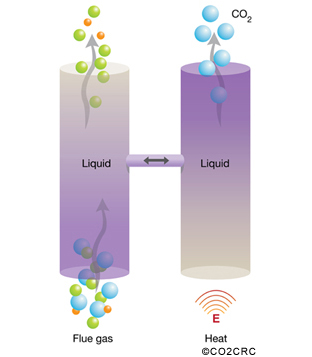
Membranes, made of polymers or ceramics, can be used to effectively sieve out carbon dioxide from gas streams. The membrane material is specifically designed to preferentially separate the molecules in the mixture. A range of configurations exists either simply as gas separation devices or incorporating liquid absorption stages. This process has not yet been applied on a large scale and there are challenges related to the composition and temperature of the flue gases.
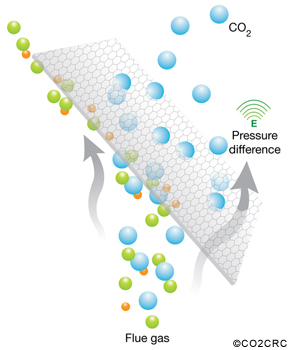
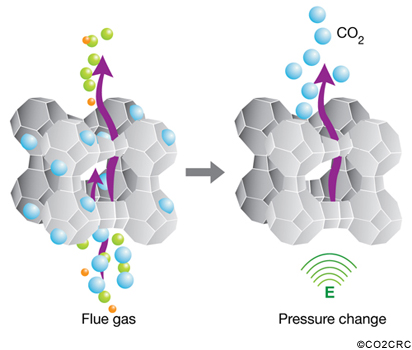
Adsorption is based on a cyclical process in which carbon dioxide is adsorbed from a gas stream on to the surface of a solid, typically a mineral zeolite. The gas stream, with most of the carbon dioxide removed, is then emitted to the atmosphere. The solid is then purified in stages using differences in either pressure or temperature to remove the carbon dioxide and compress it for storage.
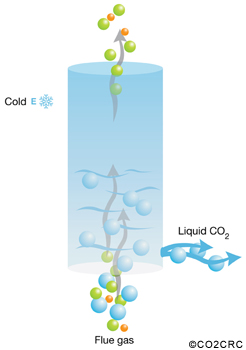
Cryogenic techniques use low temperatures to cool, condense and purify carbon dioxide from gas streams. They have been applied to moderately concentrated carbon-dioxide streams.
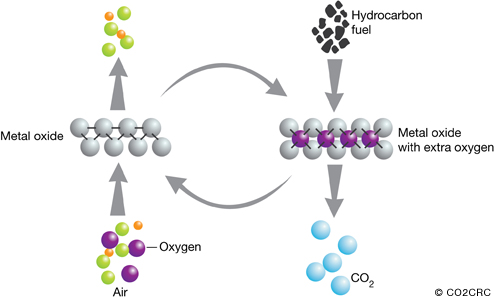
Chemical looping is similar in some ways to the oxyfiring approach in that, before combustion, oxygen is removed from air by reacting with metal particles in a fluidised bed to form metal oxides. This captured oxygen in the form of metal oxide is then contacted with the fuel, such as natural gas, in a separate fluidised bed, effectively burning the fuel and producing carbon dioxide and water.
Application
Source: Cooperative Research Centre for Greenhouse Gas Technologies (CO2CRC) in Australian
<< Previous page
---
Next page >>
TOP
|





